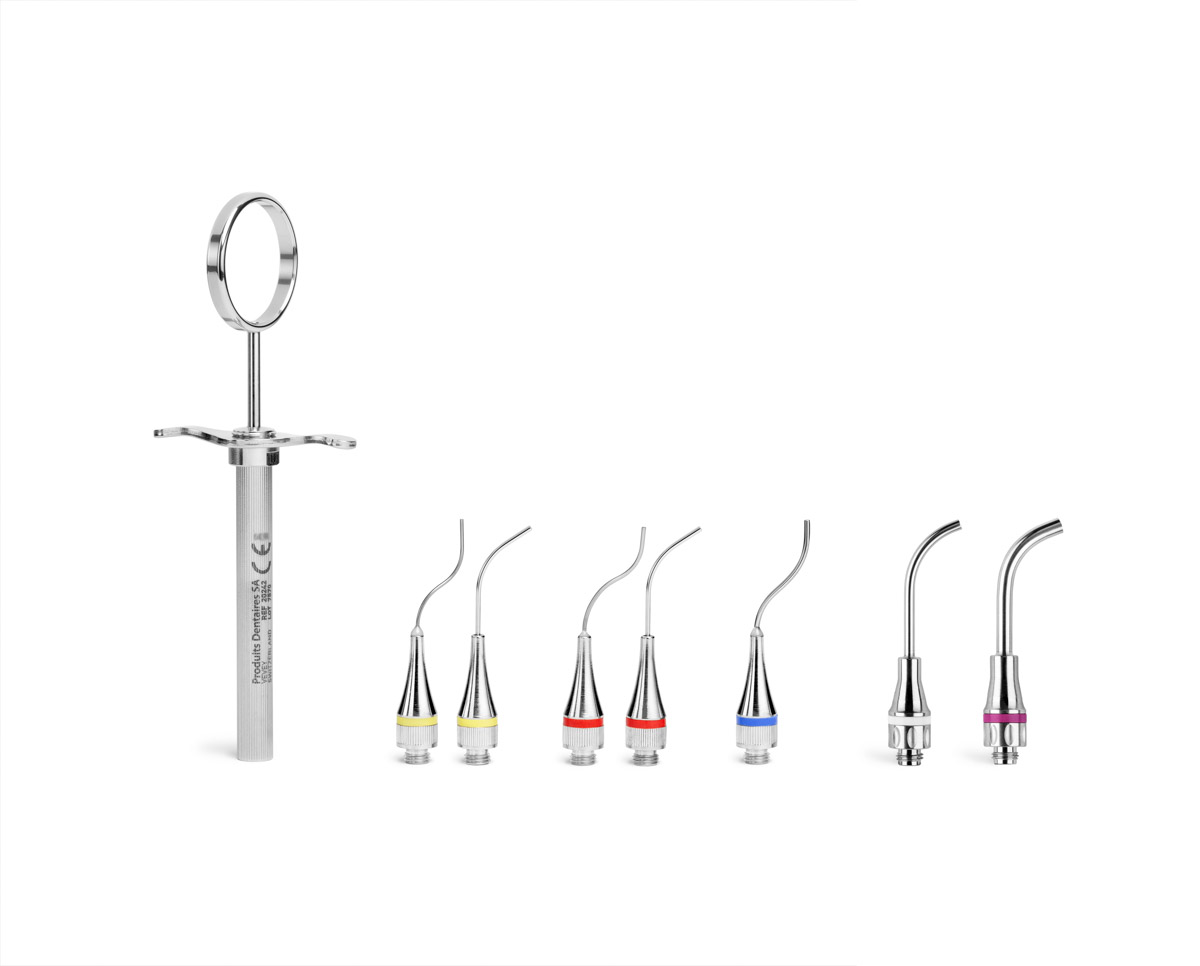
MAP System®
Endodontic obturations and repairs made simple
Content and packaging
Kits or Refills
Endodontic obturations and repairs made simple with the right placer for the perfect biocompatible material for obturation and repair.
| REF | SUGGESTED RETAIL PRICE | ||
|---|---|---|---|
| MAP One | 20296 | 329 CHF | |
| Intro Kit | 20285 | 599 CHF | |
| Universal Kit | 20286 | 849 CHF | |
| Surgical Kit | 20287 | 999 CHF | |
| MAP VPT Kit | 20288_PP | 329 CHF |
| REF | SUGGESTED RETAIL PRICE | ||
|---|---|---|---|
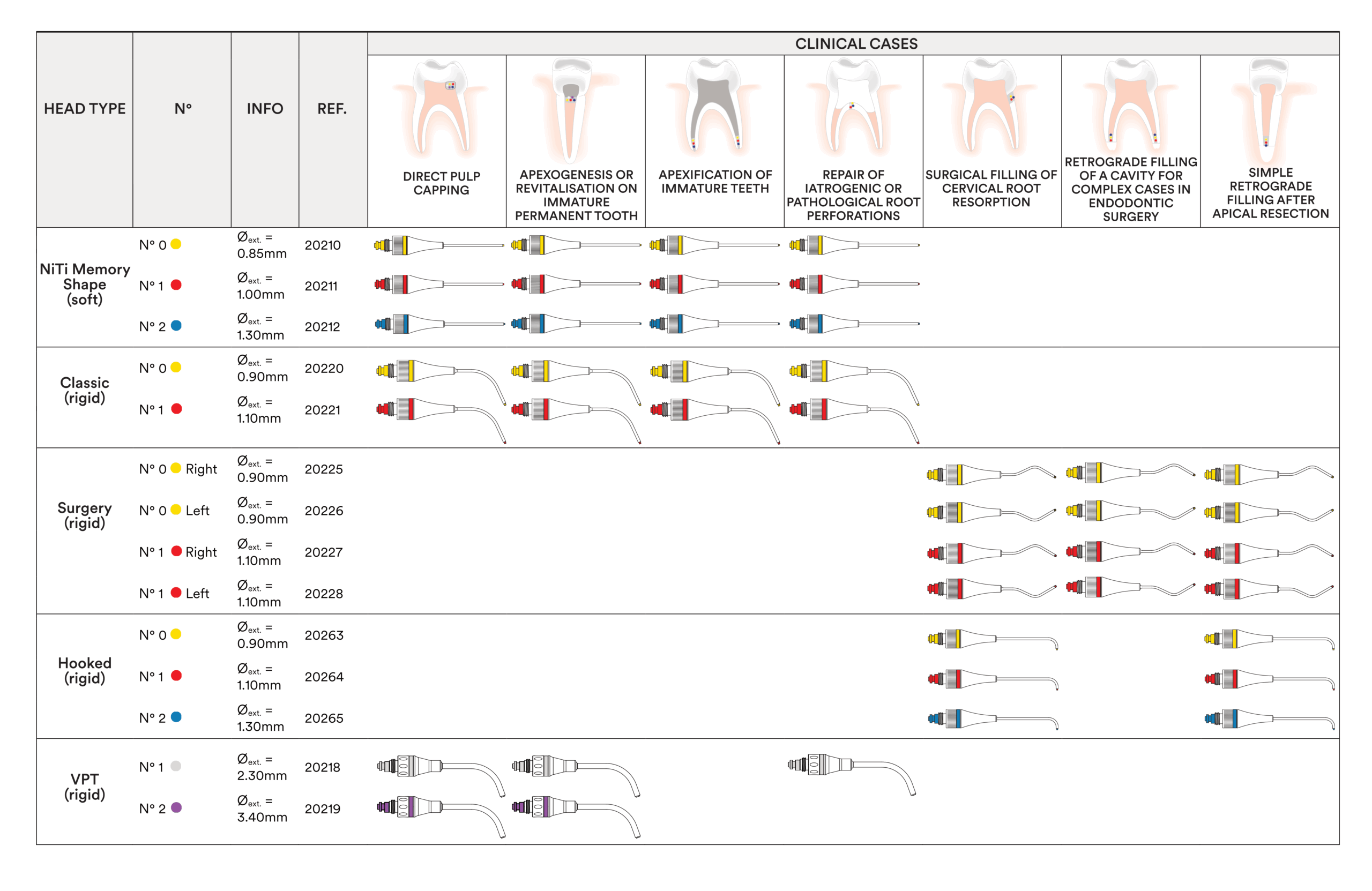
| Map Needle | Classic N°0 Yellow | 20220 | 149.35 CHF |
| Map Needle | Classic N°1 Red | 20221 | 149.35 CHF |
| Map Needle | Hooked N°0 Yellow | 20263 | 154.50 CHF |
| Map Needle | Hooked N°1 Red | 20264 | 154.50 CHF |
| Map Needle | Hooked N°2 Blue | 20265 | 154.50 CHF |
| Map Needle | Niti memory shape N°0 Yellow | 20210 | 175.10 CHF |
| Map Needle | Niti memory shape N°1 Red | 20211 | 175.10 CHF |
| Map Needle | Niti memory shape N°2 Blue | 20212 | 175.10 CHF |
| Map Needle | Surgery Right N°0 Yellow | 20225 | 154.50 CHF |
| Map Needle | Surgery Left N°0 Yellow | 20226 | 154.50 CHF |
| Map Needle | Surgery Right N°1 Red | 20227 | 154.50 CHF |
| Map Needle | Surgery Left N°1 Red | 20228 | 154.50 CHF |
| Map Needle | VPT N°1 White | 20218_PP | 175.10 CHF |
| Map Needle | VPT N°2 Purple | 20219_PP | 175.10 CHF |
| REF | SUGGESTED RETAIL PRICE | ||
|---|---|---|---|
| O-Rings | N°0 Yellow | 20252 | 31.95 CHF |
| O-Rings | N°1 Red | 20253 | 31.95 CHF |
| O-Rings | N°2 Blue | 20254 | 31.95 CHF |
| Plungers | N°0 Yellow | 20200 | 16.00 CHF |
| Plungers | N°1 Red | 20201 | 16.00 CHF |
| Plungers | N°2 Blue | 20202 | 16.00 CHF |
| Plungers | N°1 White | 20203 | 16.00 CHF |
| Plungers | N°2 Purple | 20204 | 16.00 CHF |
| CleanyFloss | Pack of 30 threads | 20249 | 9.00 CHF |
| VPT Cleaning brush | 1 cleaning brush | 20289 | 9.50 CHF |
| Stainless steel well | 1 stainless steel well | 20250 | 54.60 CHF |
NEW MAP VPT
Vital Pulp Therapy
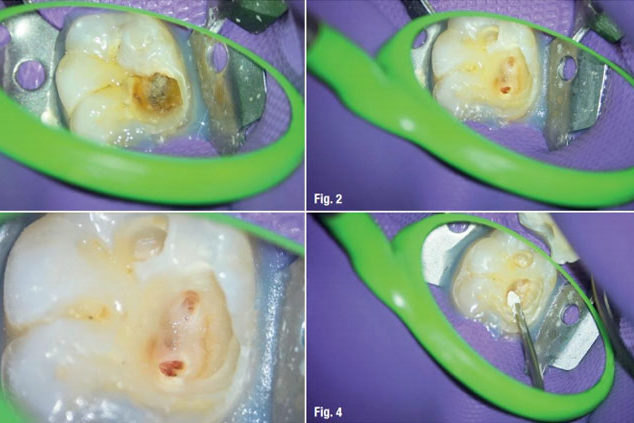
MAP VPT is an extension of the MAP SYSTEM range specifically designed for vital pulp therapy, such as pulp capping, and is particularly suitable not only for pediatric dentists and endodontists but also for general practitioners. It allows for the quick and precise placement of biomaterials for a reproducible procedure.
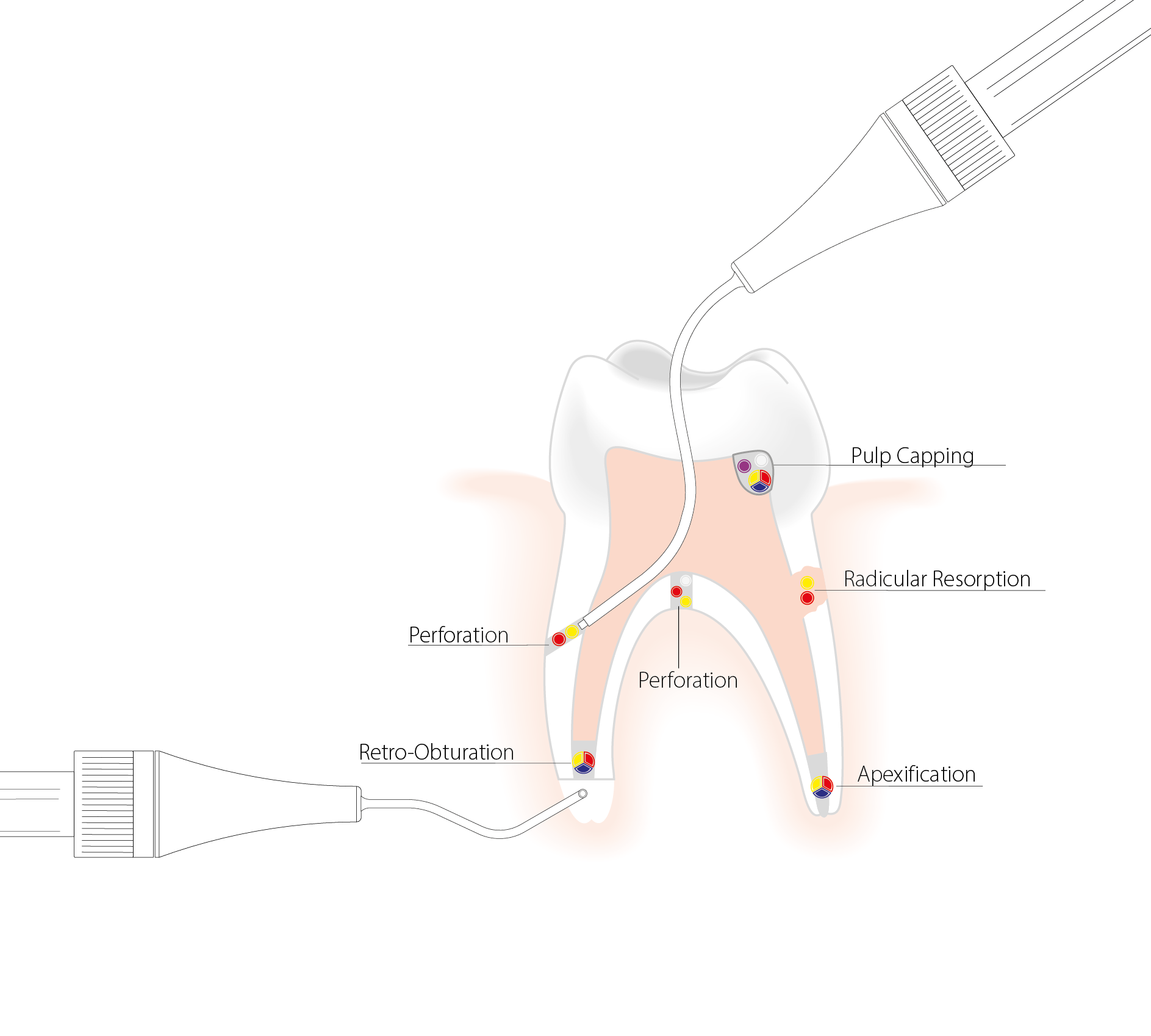
Micro Apical Placing
The MAP System significantly improves the access to the cavity and other anatomically difficult regions to reach, thanks to the various geometries of needles. The precision of placement of the cylinder of compacted MTA loaded in the needle prevents the dispersion of the material in the root canal. The MAP System is the answer to your MTA placement challenges in most clinical indications.
The MAP System is an autoclavable and reusable surgical instrument. The single-use plastic plungers are also autoclavable, allowing a complete decontamination of the entire device.
A tool at the service of precision
The MAP System is made of different needles adapted to the procedure, the size of the canals and the volume of materials required. The MAP VPT is an extension of the MAP System® range, designed for precise placement of materials in Vital Pulp Therapy.
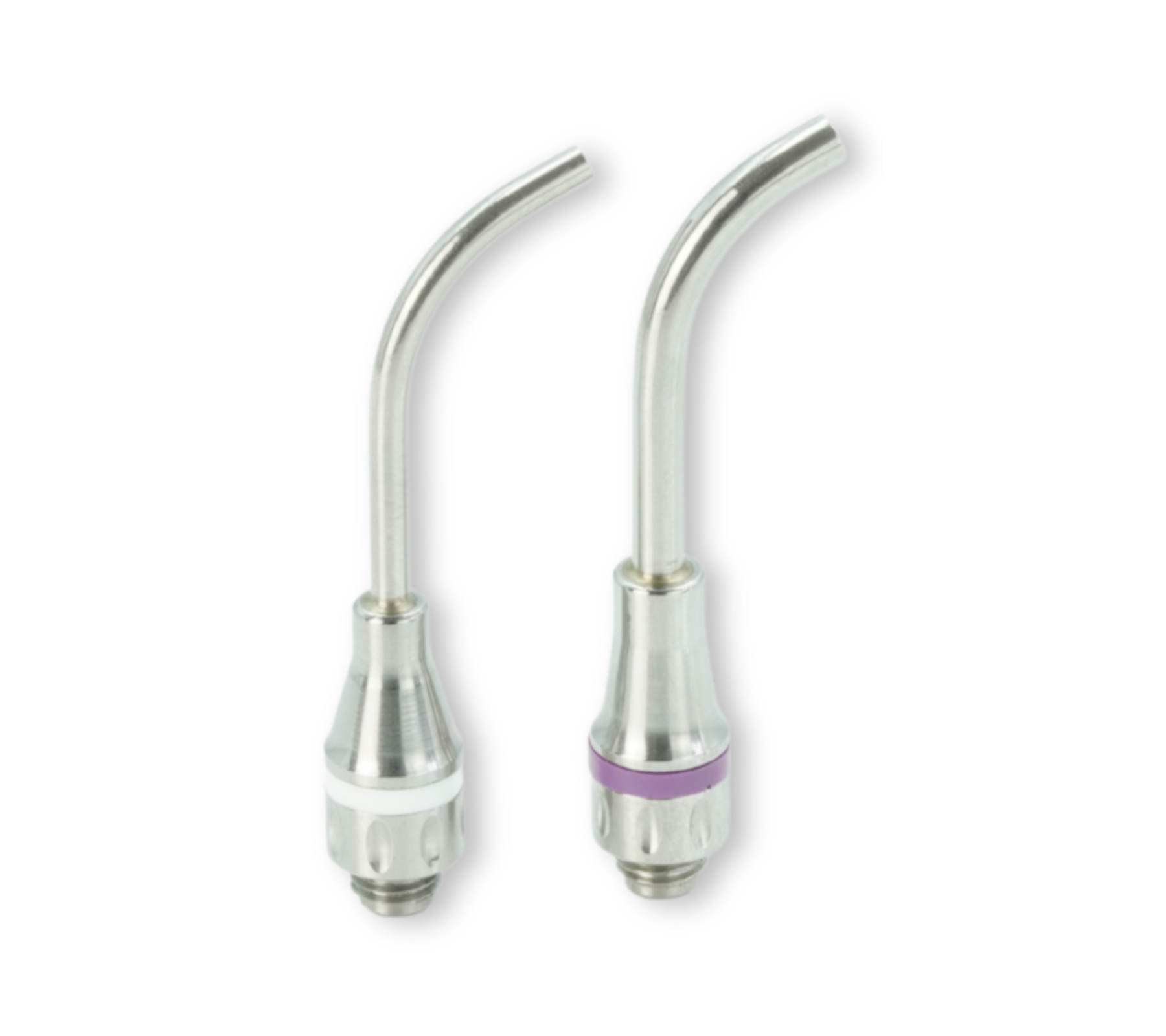
MAP System needles are available in 3 different sizes and are colour-coded: yellow = No. 0, red = No. 1, blue = No. 2. For the new MAP VPT needles, there’s a white VPT n°1 needle (diameter 2.3 mm) and a violet VPT n°2 needle (diameter 3.4 mm). The shape of the needles is specific for each clinical indication. Classic curved needles are designed for precise endodontic procedures performed using the orthograde approach. NiTi Memory Shape needles can be bent to your specific needs. Triple-angle Surgery needles and Hooked needles are intended for retrograde endodontic procedures.
The indications for MAP VPT are the placement of repair materials during pulp capping procedures, for example in cases of partial or total pulpotomy, or for pulp floor perforations.

Buy MAP System
4 kits to fit your practice
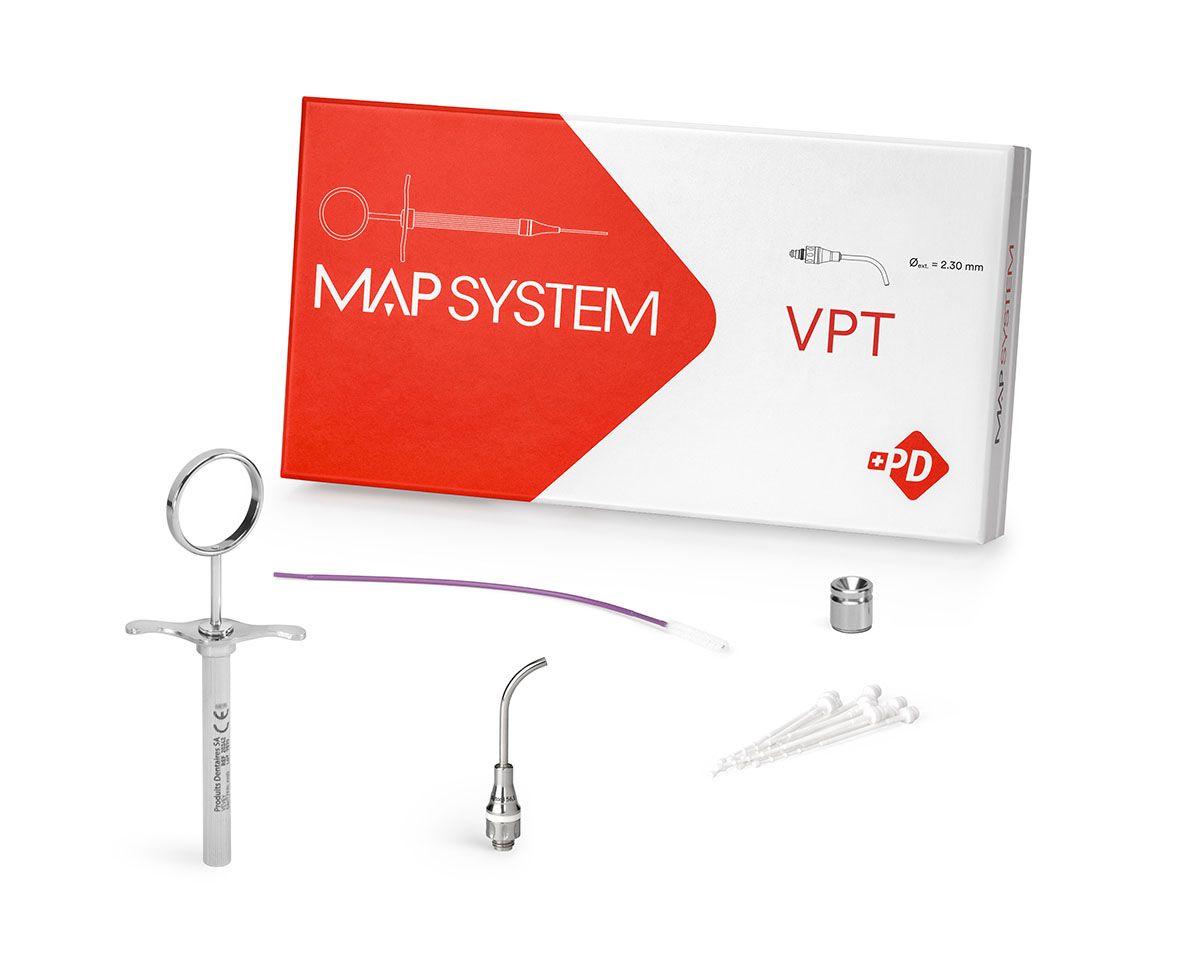
MAP VPT Kit (1 needle)
REF 20288
For precise and predictable material delivery in Vital Pulp Therapy.
- 1 White Needle, No. 1
- 1 Stainless steel syringe
- 16 white plastic pistons, No. 1
- 1 Cleaning brush
- 1 Stainless steel well
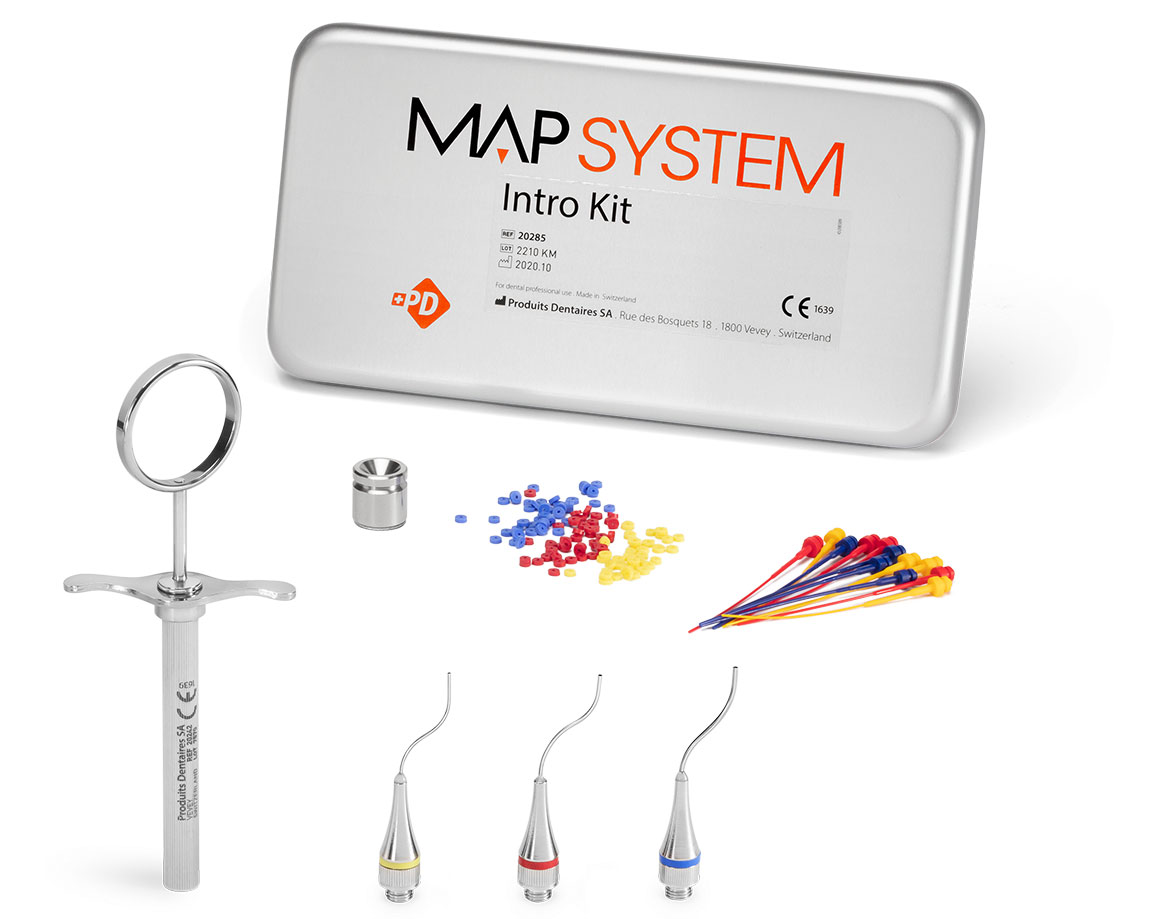
Intro Kit (3 needles)
REF 20285
The optimal kit for endodontists looking for flexibility and precision.
- 1 NiTi Memory Shape Needle, No. 0
- 1 NiTi Memory Shape Needle, No. 1
- 1 NiTi Memory Shape Needle, No. 2
- 1 Stainless steel syringe
- 8 Plastic pistons, No. 0
- 8 Plastic pistons, No. 1
- 8 Plastic pistons, No. 2
- 1 Cleaning curette, No. 0
- 1 Cleaning curette, No. 1
- 1 Cleaning curette, No. 2
- 1 Cleaning ring set, No. 0
- 1 Cleaning ring set, No. 1
- 1 Cleaning ring set, No. 2
- 1 Stainless steel well
- 1 Aluminium box 185 x 95 x 30 mm
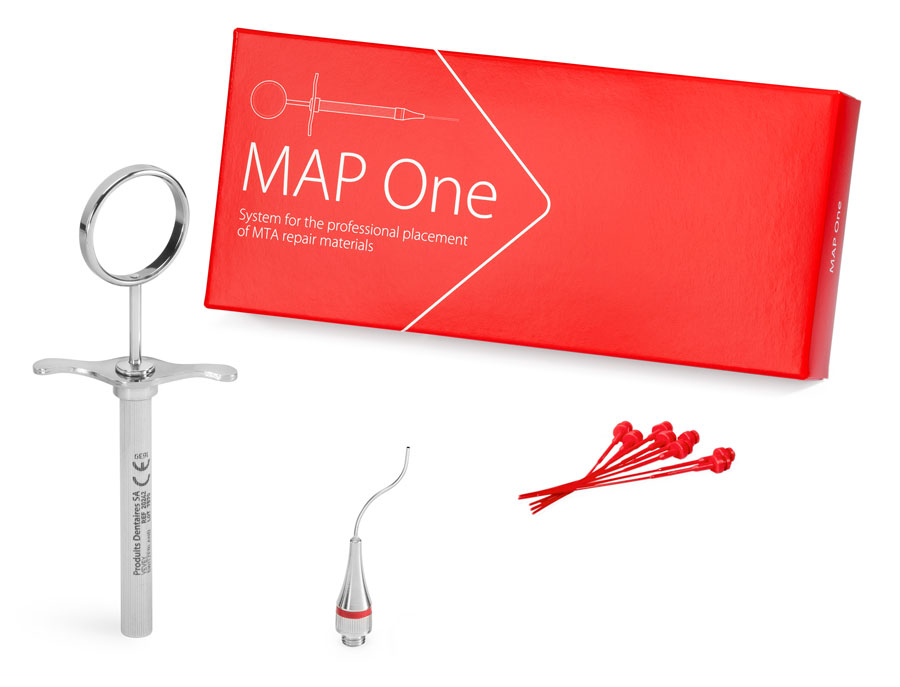
MAP One (1 needle)
REF 20296
The simplified kit for clinicians who want to acquire an efficient tool for the placement of filling materials.
- 1 NiTi Memory Shape Needle, No. 1
- 1 Stainless steel syringe
- 8 Plastic pistons, No. 1
- 1 Cleaning curette, No. 1
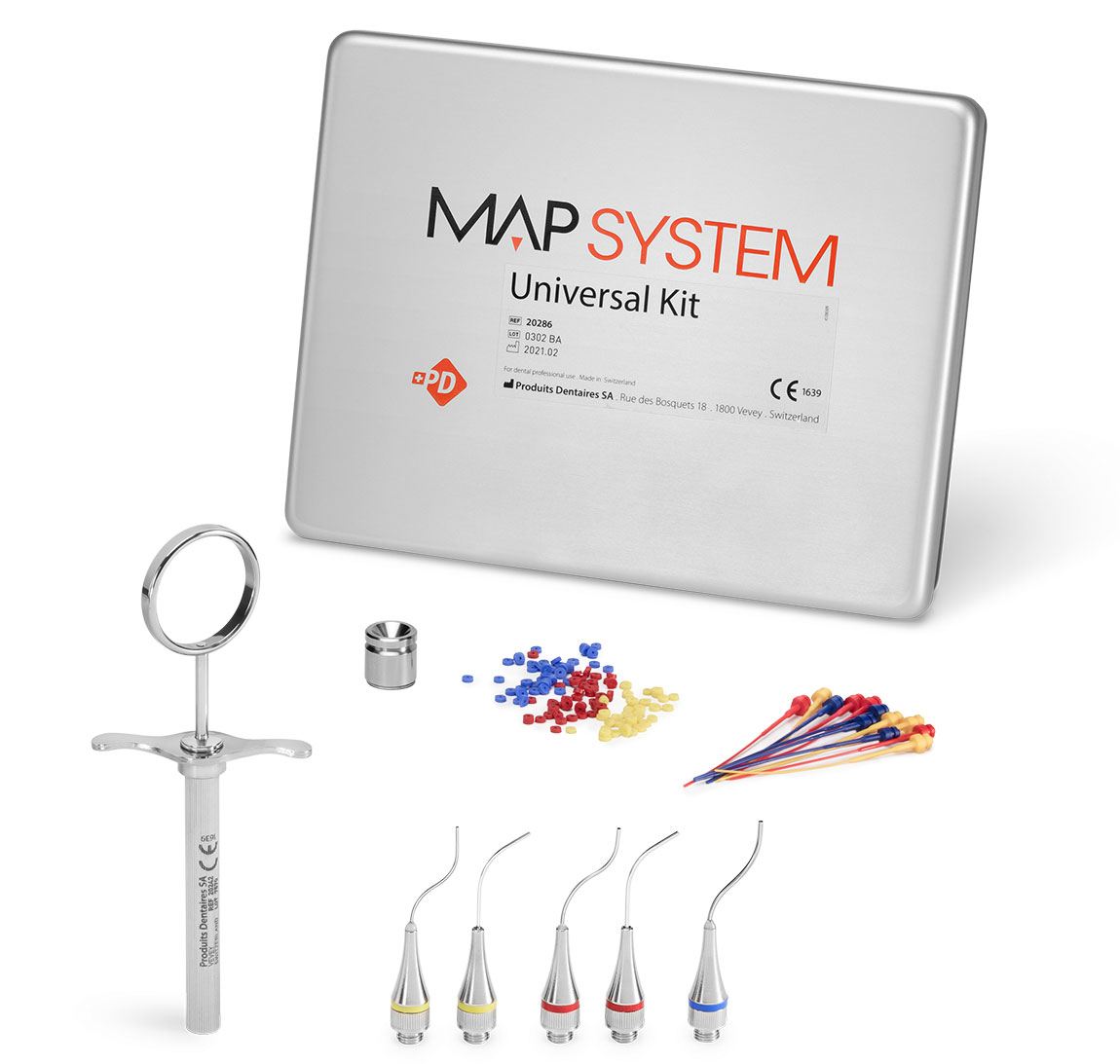
Universal Kit (5 needles)
REF 20286
The complete kit for frequent users of the MAP System who need maximum access and placement possibilities.
- 1 NiTi Memory Shape Needle, No. 0
- 1 NiTi Memory Shape Needle, No. 1
- 1 NiTi Memory Shape Needle, No. 2
- 1 Classic Needle, No. 0
- 1 Classic Needle, No. 1
- 1 Stainless steel syringe
- 16 Plastic pistons No. 0
- 16 Plastic pistons, No. 1
- 8 Plastic pistons, No. 2
- 1 Cleaning curette, No. 0
- 1 Cleaning curette, No. 1
- 1 Cleaning curette, No. 2
- 1 Cleaning ring set, No. 0
- 1 Cleaning ring set, No. 1
- 1 Cleaning ring set, No. 2
- 1 Stainless steel well
- 1 Aluminium box 185 x 145 x 30 mm
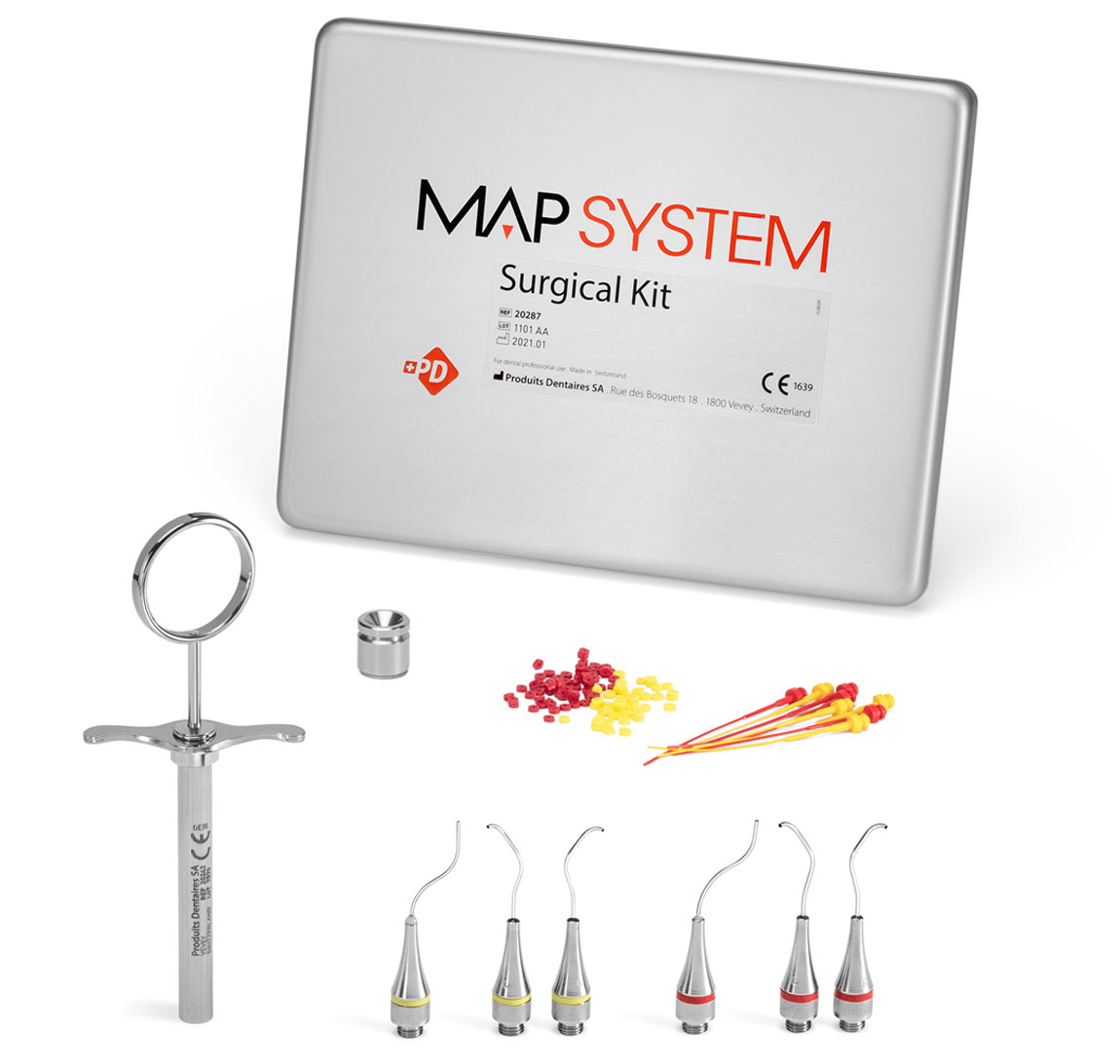
Surgical Kit (6 needles)
REF 20287
The specific kit for surgical specialists encountering complex cases of retrograde filling.
- 1 Surgery Needle, No. 0, right
- 1 Surgery Needle, No. 0, left
- 1 Surgery Needle, No. 1, right
- 1 Surgery Needle, No. 1, left
- 1 NiTi Memory Shape Needle, No. 0
- 1 NiTi Memory Shape Needle, No. 1
- 1 Stainless steel syringe
- 24 Plastic pistons, No. 0
- 24 Plastic pistons, No. 1
- 1 Cleaning curette, No. 0
- 1 Cleaning curette, No. 1
- 1 Cleaning ring set, No. 0
- 1 Cleaning ring set, No. 1
- 1 Stainless steel well
- 1 Aluminium box 185 x 145 x 30 mm
Testimonials

“I saw the MAP system for the first time at the exhibition of the AAE several years ago and I think I have been the first Italian Endodontist who started using it. It has been a “love at first sight”. Since then I use the MAP System in every case where it is indicated the use of MTA. It is the best MTA carrier available in the market for both nonsurgical and surgical endodontics. More recently I had the opportunity to try and use the new PD White MTA, which has the great advantage of setting in a very fast time and it is not causing discoloration when used to repair buccal perforation in anterior teeth. I recommend the use of MAP System and of the new formula of PD White MTA to all the colleagues who want to obtain excellent results for their patients.”

“MAP system provides a unique and efficient method for precisely placing MTA and also other bioceramic materials. In my clinical experience, Map system is the perfect device and the best choice for perforation management, apical plug, pulp capping and retro- obturation for micro apical surgery. It makes the procedure more predictable and easier to handle thanks to different size of tips and the NiTi Memory Shape. The flexibility of the tips makes it easy for the operator to bundle manually to fit to the shape of the root canal for effective placement of the bioceramic material. I personally strongly recommend Map One System to all colleagues, easy manipulation and effective for all procedures mentioned above.”

“MAP System is simply amazing. It allows clinicians to pre-bend the NiTi carrier and place MTA without any problems since the plugger inside the tube is plastic and smoothly follows the curvature. I find MAP system remarkable, not only for the intra-canal use, but also for pulp capping procedures that demand precision and confidence. It’s simply genius !”

“I have been using MAP System formany years for MTA placement. Its clinical versatility is incomparable, especially due to the NiTi bendable tips that allow the best ergonomy in delivering the material in the most challenging scenarios.”

“Thanks to the MAP System, I have the possibility to achieve a perfect retrograde filling of 4 mm deep without overflow. The quality of the filling is significantly improved. The triple angle needles provide excellent access and a perfect overview of the surgical field.”

“The flexibility of NiTi needles is a real added value. The various MAP System kits are dedicated not only to endodontic specialists, but will also be very useful for general practitioners who may need to use these devices in their daily practice.”
“When you are able to place MTA exactly where it is needed, you finally understand how essential the Map System is.”

“Pulp vitality preservation strategies are an integral part of modern dentistry. They require not only knowledge in pulp biology and biomaterials but also a solid mastery of the practical treatment procedure, such as the optimal placement of the capping material. In this regard, the new VPT tips of the MAP System are tools that make the procedure more precise, easier, predictable and therefore more reliable.”
Where to buy?
Find a dealer
Please select your country from the list below.
Video presentation
Cleaning Instructions
Video presentation MAP VPT
Associated products
PDTM MTA White
The PDTM MTA White is an aggregate of mineral trioxide whose opacifier, calcium tungstate, avoids the risks of stains and discolouration sometimes encountered with bismuth oxide. The optimised particle size distribution of its fine hydrophilic powder contributes to a quick and easy mixing. Its short setting time, around 15 minutes, makes it possible to carry out the restoration almost immediately. Humidity does not affect its mechanical characteristics in any way. Its low solubility creates a good seal, and its excellent marginal sealing ability prevents the migration of fluids into the root canal. The alkaline character of cement gives it antibacterial properties, and its hydrophilic character promotes the neoformation of cement by creating a favourable environment for the healing of periodontal tissues. It also stimulates the formation of a dentinal barrier directly in contact with the pulp.
The excellent biocompatibility of PDTM MTA White with the dentine wall and pulp allows its use for orthograde and retrograde root fillings, to treat cervical root resorptions as a pulp capping material, as well as for the apexification and pulpotomy of immature teeth in children and adolescents.


CleanyFloss
Extra-fine nylon fleece threads equipped with a threading aid, designed for thorough and effective cleaning of braces, implants, bridges, brackets, and wider interdental spaces. High tensile strength. Perfect for the pre-cleaning of the MAP System device.
News about this specific product
* This product is a medical device of Class IIa and fulfills the applicable requirements of the Directive 93/42/EEC and of the Regulation 2023/607 on medical devices. This Medical Device is covered by SGS Belgium NV (Notified Body number 1639) accreditation as mentioned in the Declaration of Conformity.
** Product availability may depend on your local regulation and product registration status.