Opacal
Pre-mixed Calcium Hydroxide paste
Content and packaging
Syringe of 3 ml with 10 applications tips and 1 finger grip.
Description
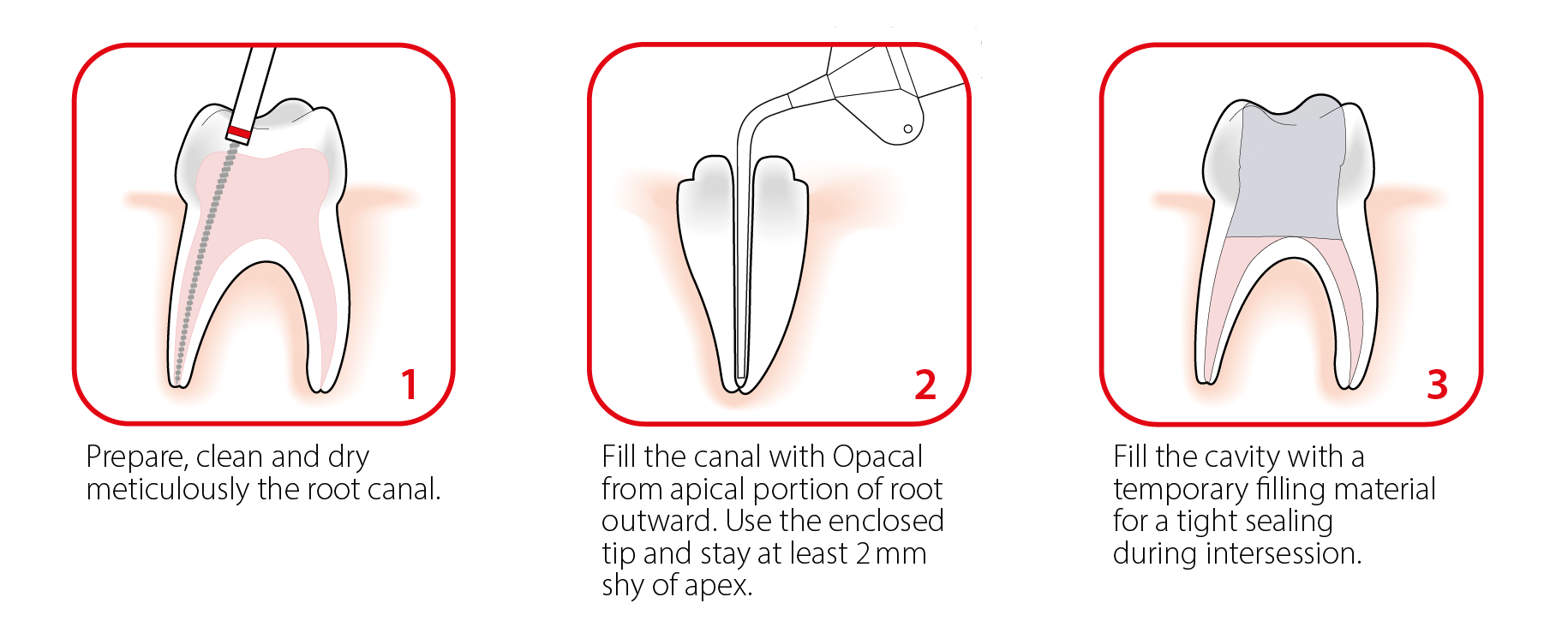
Opacal is a radiopaque, creamy and ready-to-use calcium hydroxide paste for temporary root canal dressing. When temporisation of the root canal between two appointments is required, calcium hydroxide paste is the standard material to use. The product is intended to eliminate remaining microorganisms and prevent reinfection between. The main properties of the calcium hydroxide paste are thanks to its alkaline pH. The usual duration of temporisation treatment is between 1 and 3 weeks.
The product features a high pH (above 12). With a radiopacity > 300% of the dentine radiopacity, Opacal is one of the most radiopaque calcium hydroxide paste, ensuring easy identification and efficient follow-up. Opacal is an aqueous non-setting material. This characterisitic induces rapid ionic release and performant action in short treatment time procedure (R.G. Fava & W.P. Saunder – Calcium hydroxide pastes: classification and clinical indications). In addition, the paste can be easily removed.
Opacal is packaged in a 3ml syringe and provided with 10 dedicated application tips and a finger grip. The combined use of ready-to-use paste and luer-lock application tip enable to deliver the paste in the root canal anatomy with safety and precision. The dedicated tips are flexible and designed to reach desired zone of different root canal anatomy. The finger grip plate provided with the device enables to control more precisely the handling and delivery of the paste with finger. Additional application tips can be ordered with dedicated reference.
Product references
| Size | REF | SUGGESTED RETAIL PRICE | |
|---|---|---|---|
| Syringe of 3 ml | Kit | 11020 | 23.90 CHF |
| 10 Application Tips | Refills | 11022 | 8.00 CHF |
* This product is a medical device of Class IIa and fulfills the applicable requirements of the Directive 93/42/EEC on medical devices. This Medical Device is covered by SGS Belgium NV (Notified Body number 1639) accreditation as mentioned in the Declaration of Conformity.


